
Data from Phase 3 clinical trials, SKYLIGHT 1 and 2 and DAYLIGHT, demonstrated improvements in several patient-reported outcome measures of sleep disturbance with fezolinetant treatment
SKYLIGHT 1 and 2 individual trial results
Figure 1. Change in PROMIS SD SF 8b total score from Baseline to Weeks 4 and 12 in SKYLIGHT 1 and SKYLIGHT 29,10
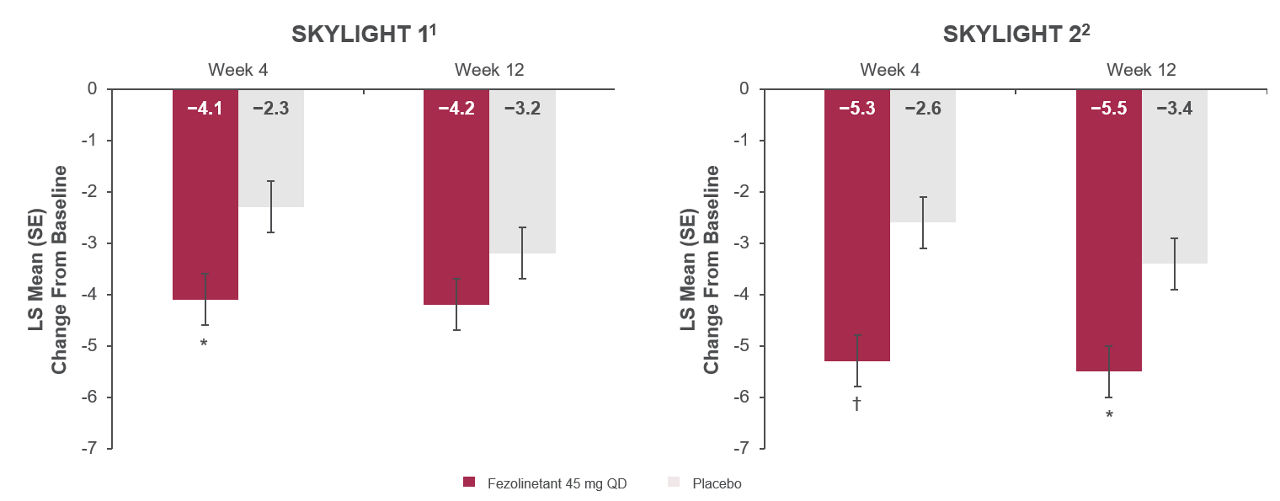
*P<0.01 vs placebo. †P<0.001 vs placebo.
Mean (SD) Baseline values in SKYLIGHT 1: fezolinetant 45 mg, 27.1 (7.0); placebo, 26.4 (6.6).
Mean (SD) Baseline values in SKYLIGHT 2: fezolinetant 45 mg, 26.2 (6.6); placebo 27.4 (7.0).
All randomized participants assessed according to randomization at first dose (SKYLIGHT 1: placebo n = 175, fezolinetant 45 mg n = 174; SKYLIGHT 2: placebo n = 167, fezolinetant 45 mg n = 167).
Adapted from: Lederman S, Lancet 2023 and Johnson KA, J Clin Endocrinol Metab. 2023
SKYLIGHT 1 and 2 trial pooled analysis results
Figure 2. Change in PROMIS SD SF 8b total score from Baseline to Weeks 4 and 121
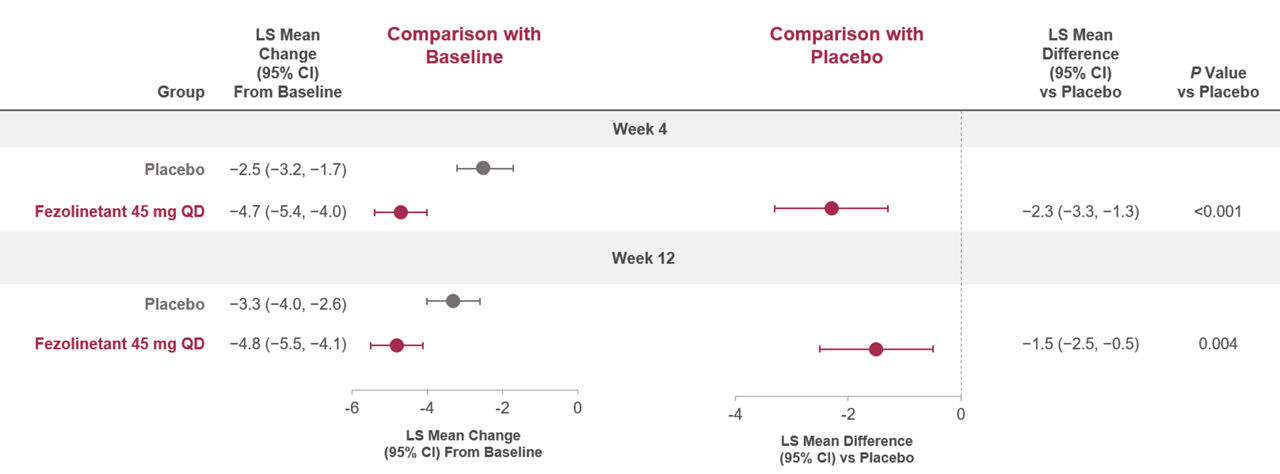
Baseline data (mean [SD]): placebo (26.9 [6.8]), fezolinetant 45 mg (26.7 [6.8]). A negative change indicated a reduction from Baseline (i.e., a favorable outcome).
All randomized participants assessed according to randomization at first dose (fezolinetant 45 mg: n = 341, placebo: n = 342).
Adapted from: Shapiro M, Maturitas 2024.
Figure 3. Change in PROMIS SRI SF 8a total score from Baseline to Weeks 4 and 121
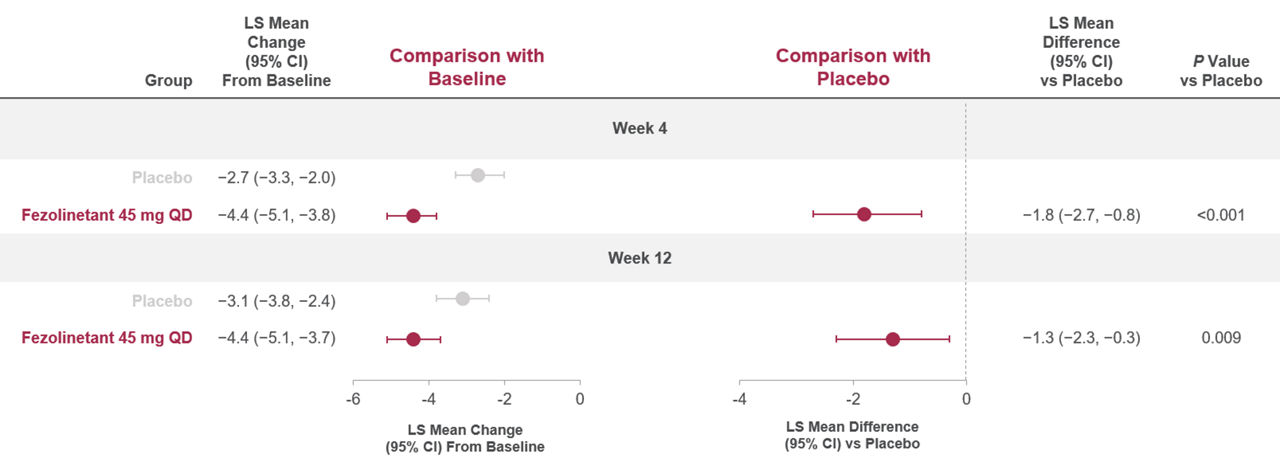
Baseline data (mean [SD]): placebo (21.6 [7.4]), fezolinetant 45 mg (21.7 [7.2]). A negative change indicated a reduction from Baseline (i.e., a favorable outcome).
All randomized participants assessed according to randomization at first dose (fezolinetant 45 mg: n = 341, placebo: n = 342).
Adapted from: Shapiro M, Maturitas 2024.
Figure 4. Distribution of the PGI-C SD at Weeks 4 and 121
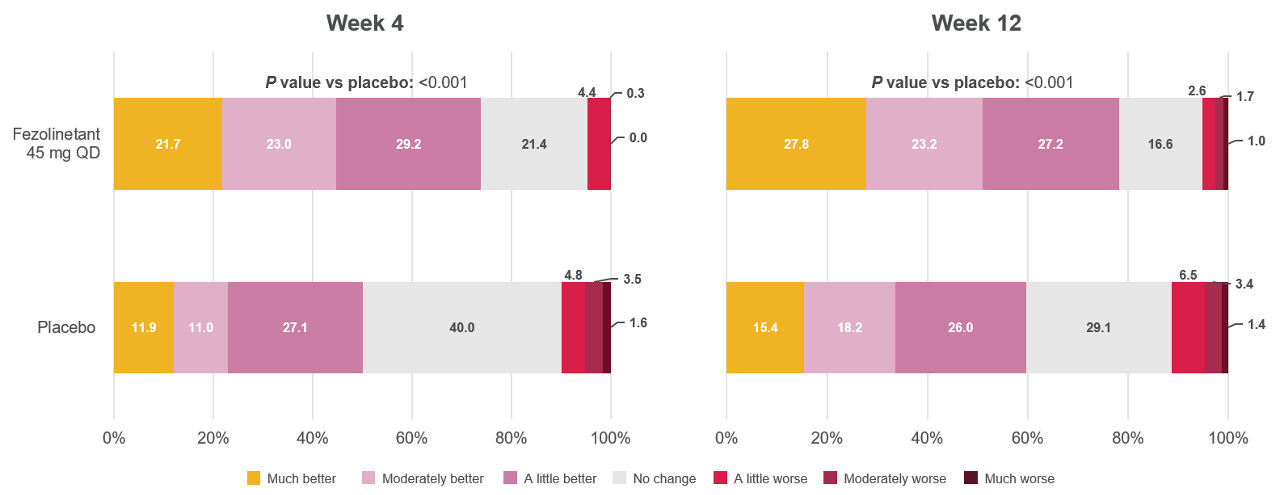
All randomized participants assessed according to randomization at first dose (fezolinetant 45 mg: n = 341, placebo: n = 342).
Adapted from: Shapiro M, Maturitas 2024.
Figure 5. Distribution of the PGI-S SD at baseline, Week 4, and Week 121
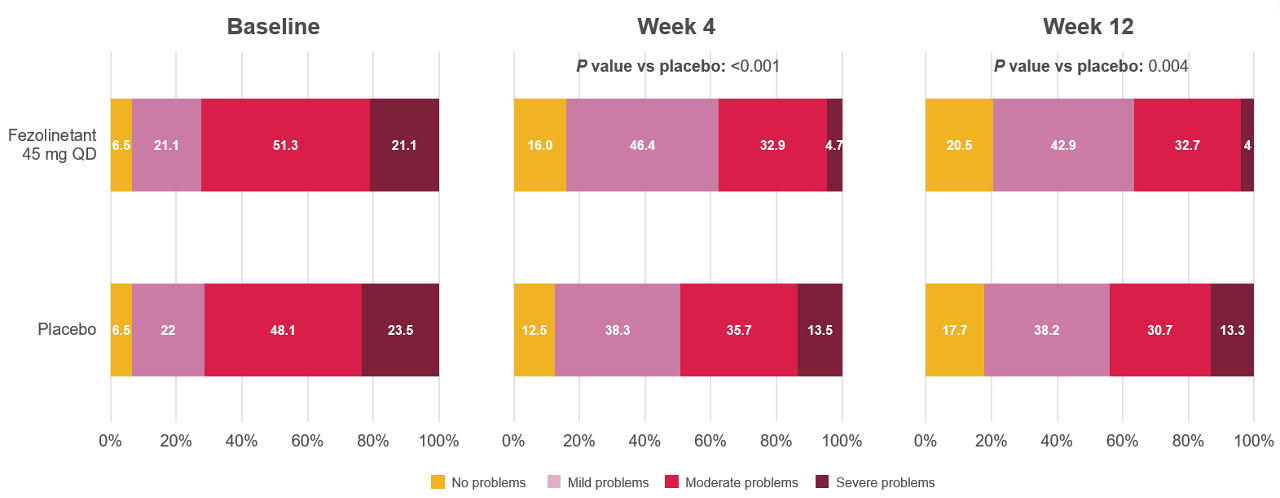
All randomized participants assessed according to randomization at first dose (fezolinetant 45 mg: n = 341, placebo: n = 342).
Adapted from: Shapiro M, Maturitas 2024.
DAYLIGHT trial results
Figure 6. Change in PROMIS SD SF 8b total score from baseline to Week 242,11
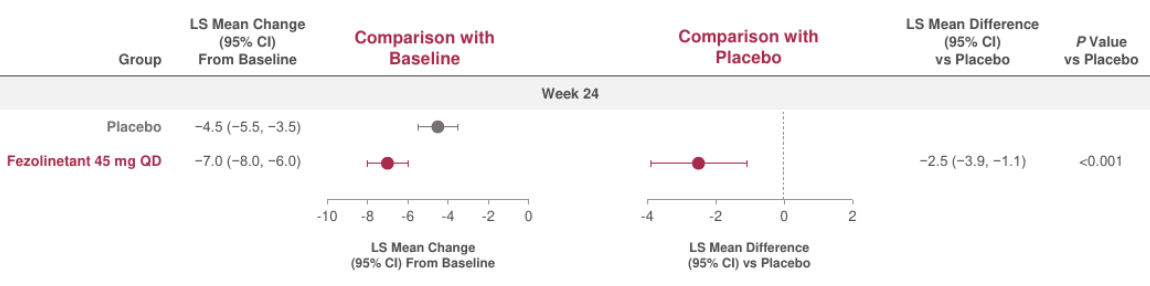
Baseline data (mean [SD]): placebo (27.6 [6.3]), fezolinetant 45 mg (28.3 [6.1]).
All randomized participants assessed according to randomization at first dose (placebo: n = 226, fezolinetant 45 mg: n = 226).
A negative change indicated a reduction from baseline (i.e., a favorable outcome).
Adapted from: Schaudig K, BMJ 2024.
Figure 7. Distribution of PGI-C SD at Week 242
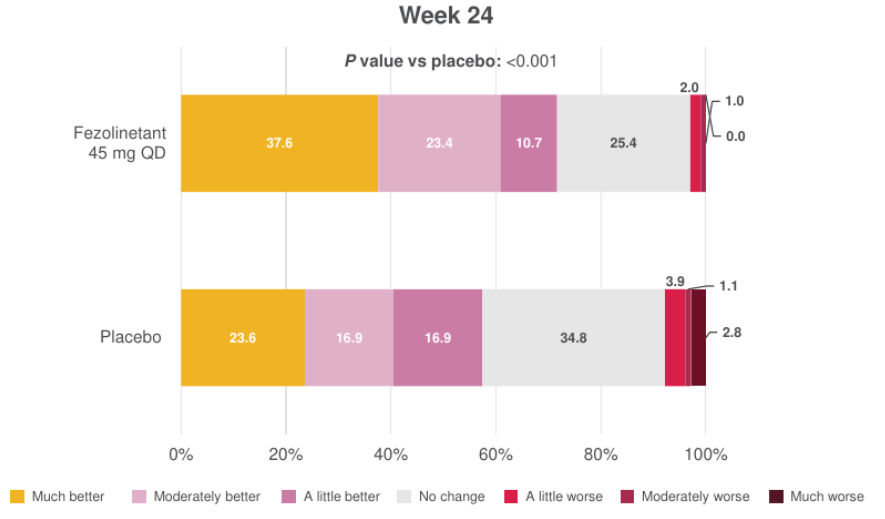
All randomized participants assessed according to randomization at first dose (fezolinetant 45 mg: n = 226, placebo: n = 226).
Adapted from: Schaudig K, BMJ 2024.
Figure 8. Distribution of PGI-S SD at baseline and Week 243
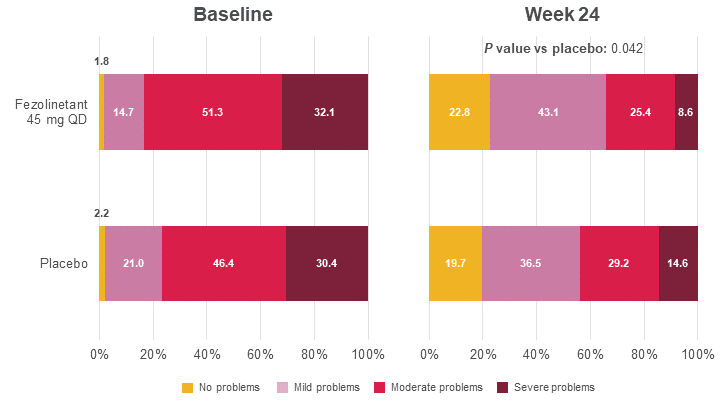
All randomized participants assessed according to randomization at first dose (fezolinetant 45 mg: n = 226, placebo: n = 226).
Adapted from: Shapiro M, Maturitas 2024.
1. Shapiro M, Cano A, Nappi RE, et al. Effect of fezolinetant on sleep disturbance and impairment during treatment of vasomotor symptoms due to menopause. Maturitas. 2024; Available at: https://doi.org/10.1016/j.maturitas.2024.107999.
2. Schaudig K, Wang X, Bouchard C, et al. Efficacy and safety of fezolinetant for moderate-severe vasomotor symptoms associated with menopause in individuals unsuitable for hormone therapy: phase 3b randomized controlled trial. BMJ. 2024;(387):e079525. Available at: https://doi.org/10.1136/bmj-2024-079525.
3. Shapiro M, Wu X, Wang X, et al. Effect of fezolinetant on patient-reported quality-of-life outcomes: Data from a phase 3b study (DAYLIGHT) of the treatment of moderate to severe vasomotor symptoms associated with menopause in women considered unsuitable for hormone therapy. Maturitas. 2024; Available at: https://doi.org/10.1016/j.maturitas.2024.108159.
4. Rayment E, Kemp C, Freire M, et al. Trial-in-progress: Real-world sleep outcomes among women with vasomotor symptoms associated with menopause using fezolinetant (ADELE-AU). Poster presented at 27th Australasian Menopause Society (AMS) Congress 12–14 September 2025, Fremantle, Western Australia, Australia.
5. Thurston R, Chang Y, Buysse D, et al. Hot flashes and Awakenings Among Midlife Women. Sleep. 2019;(0161-8105) Available at: https://doi.org/10.1093/sleep/zsz131.
6. English M, Stoykova B, Slota C, et al. Qualitative study: burden of menopause-associated vasomotor symptoms (VMS) and validation of PROMIS Sleep Disturbance and Sleep-Related Impairment measures for assessment of VMS impact on sleep. J. Patient-Rep. Outcomes. 2021;5(1):37. Available at: https://doi.org/10.1186/s41687-021-00289-y.
7. DePree B, Shiozawa A, King D, et al. Association of menopausal vasomotor symptom severity with sleep and work impairments: A US survey. Menopause. 2023;30(9):887-897. Available at: https://doi.org/10.1097/gme.0000000000002237.
8. Maki PM, Panay N, Simon JA. Sleep disturbance associated with the menopause. Menopause. 2024;31(8):724-733. Available at: https://doi.org/10.1097/gme.0000000000002386.
9. Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401(10382):1091-1102. Available at: https://doi.org/10.1016/s0140-6736(23)00085-5.
10. Johnson KA, Martin N, Nappi RE, et al. Efficacy and Safety of Fezolinetant in Moderate to Severe Vasomotor Symptoms Associated With Menopause: A Phase 3 RCT. J Clin Endocr Metab. 2023;108(8):1981-1997. Available at: https://doi.org/10.1210/clinem/dgad058.
11. Data on file.
The medical information on this website is for educational purposes only and is intended to provide scientific information about Astellas products. This information is not intended as medical advice or clinical recommendations. This website is for use only by United States residents and licensed healthcare professionals (HCPs) practicing in the United States. Product labeling may vary between countries.
Please choose an option that best describes you:
For visitors outside the United States: click here