
There are no suggestions from nonclinical or clinical data that rebound could be expected with fezolinetant treatment
Figure 1. Weekly mean frequency of moderate and severe VMS per 24 hours over the 52-week treatment period and follow-up period in SKYLIGHT 1 and 23–5,7
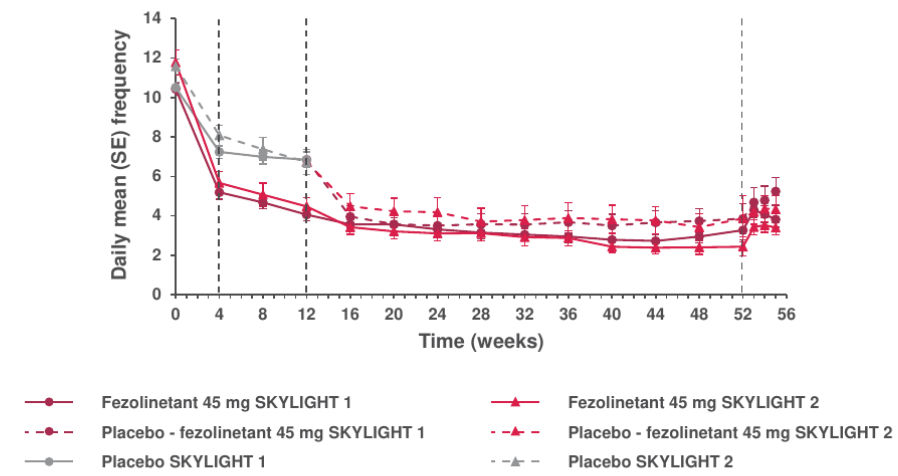
Adapted from: Johnson KA J Clin Endocr Metab. 2023, Lederman S Lancet. 2023 and Neal-Perry G TMS. 2025.
Figure 2. Weekly mean severity of moderate and severe VMS per 24 hours over the 52week treatment period and follow-up period in SKYLIGHT 1 and 23–5,7

Adapted from: Johnson KA J Clin Endocr Metab. 2023, Lederman S Lancet. 2023 and Neal-Perry G TMS. 2025.
Fraser GL, Lederman S, Waldbaum A, et al. A phase 2b, randomized, placebo-controlled, double-blind, dose-ranging study of the neurokinin 3 receptor antagonist fezolinetant for vasomotor symptoms associated with menopause. Menopause. 2020;27(4):382-392. Available at: https://doi.org/10.1097/GME.0000000000001510.
Depypere H, Timmerman D, Donders G, et al. Treatment of Menopausal Vasomotor Symptoms With Fezolinetant, a Neurokinin 3 Receptor Antagonist: A Phase 2a Trial. J Clin Endocr Metab. 2019;104(12):5893-5905. Available at: https://doi.org/10.1210/jc.2019-00677.
Neal-Perry G, Stute P, Martins K, et al. Vasomotor Symptoms due to Menopause after Treatment Discontinuation in Phase 3 Fezolinetant SKYLIGHT Studies [Poster Presentation]. Orlando, FL, USA. The Menopause Society (TMS) 2025 Annual Meeting; 2025.
Johnson KA, Martin N, Nappi RE, et al. Efficacy and Safety of Fezolinetant in Moderate to Severe Vasomotor Symptoms Associated With Menopause: A Phase 3 RCT. J Clin Endocr Metab. 2023;108(8):1981-1997. Available at: https://doi.org/10.1210/clinem/dgad058.
Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401(10382):1091-1102. Available at: https://doi.org/10.1016/s0140-6736(23)00085-5.
Neal-Perry G, Cano A, Lederman S, et al. Safety of Fezolinetant for Vasomotor Symptoms Associated With Menopause: A Randomized Controlled Trial. Obstet Gynecol. 2023;141(4):737-747. Available at: https://doi.org/10.1097/aog.0000000000005114.
Data on file.
The medical information on this website is for educational purposes only and is intended to provide scientific information about Astellas products. This information is not intended as medical advice or clinical recommendations. This website is for use only by United States residents and licensed healthcare professionals (HCPs) practicing in the United States. Product labeling may vary between countries.
Please choose an option that best describes you:
For visitors outside the United States: click here