
Phase 1 and Phase 2 studies are evaluating zolbetuximab + chemotherapy as first-line treatment for CLDN18.2-positive, metastatic pancreatic adenocarcinoma
GLEAM
Study details can be accessed using the link below:
NCT06396091
Figure 1. Clinical trial design5
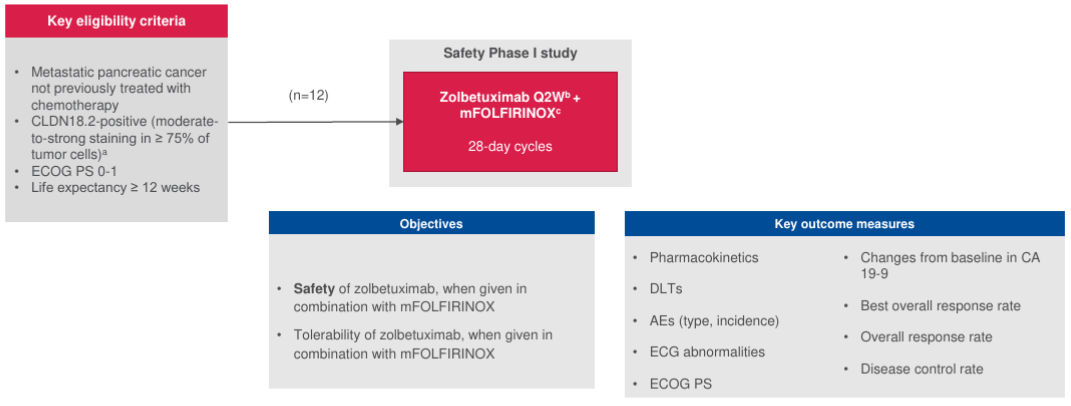
a By central IHC testing. bZolbetuximab will be administered IV on Day 1 then every two weeks. c mFOLFIRINOX will be administered IV within two days after administration of zolbetuximab.
AE, adverse event; CA 19-9, Cancer Antigen 19-9; CLDN18.2, claudin 18 isoform 2; DLT, Dose-limiting toxicity; ECG, electrocardiogram; ECOG PS, Eastern Cooperative Oncology Group performance status; IHC, immunohistochemistry; mFOLFIRINOX, modified oxaliplatin, leucovorin, irinotecan and fluorouracil; Q2W, every 2 weeks.
Astellas is unable to share additional information at this time. This Phase 1 study is currently recruiting.
Please see NCT06396091 for full study details.
The medical information on this website is for educational purposes only and is intended to provide scientific information about Astellas products. This information is not intended as medical advice or clinical recommendations. This website is for use only by United States residents and licensed healthcare professionals (HCPs) practicing in the United States. Product labeling may vary between countries.
Please choose an option that best describes you:
For visitors outside the United States: click here