
Phase 2, open-label, randomized study of zolbetuximab + chemotherapy as first-line treatment for CLDN18.2-positive, metastatic pancreatic adenocarcinoma
Figure 1. Clinical trial design3,4
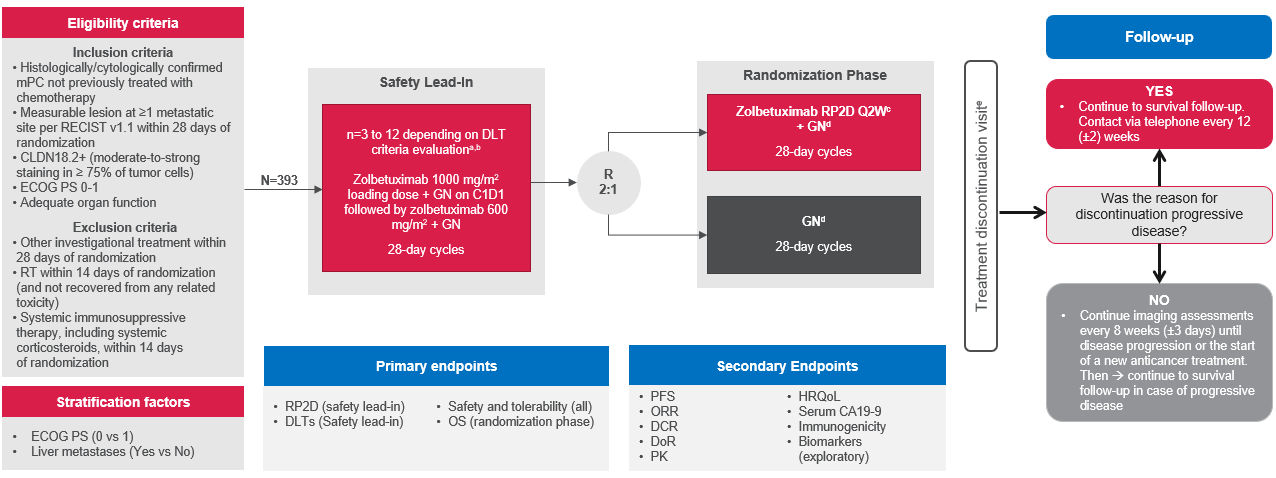
a Safety review after Cycle 1 to determine RP2D. b First three patients received zolbetuximab 1000 mg/m2 intravenously over at least 2 hours on C1D1, then 600 mg/m2 Q2W after antiemetic premedication (NK-1 receptor blockers and 5-HT3 receptor blockers recommended; corticosteroids not recommended). c Days 1 and 15 of each 28-day cycle. d Days 1, 8, and 15 of each 28-day cycle. e Patients will receive treatment until they meet the discontinuation criteria.
5-HT3, 5-hydroxytryptamine type 3; C1D1, Cycle 1 Day 1; CA19-9, cancer antigen 19-9; CLDN18.2, claudin 18.2; DCR, disease control rate; DLT, dose limiting toxicity; DOR, duration of response; ECOG PS, Eastern Cooperative Oncology Group performance status; GN, gemcitabine and nab-paclitaxel; HRQoL, health-related quality of life; mPC, metastatic pancreatic cancer; NK 1, neurokinin-1; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PK, pharmacokinetics. Q2W, every 2 weeks; RECIST, Response Evaluation Criteria in Solid Tumours; RP2D, recommended Phase 2 dose; RT, radiotherapy.
Please see NCT03816163 for full study details.
The medical information on this website is for educational purposes only and is intended to provide scientific information about Astellas products. This information is not intended as medical advice or clinical recommendations. This website is for use only by United States residents and licensed healthcare professionals (HCPs) practicing in the United States. Product labeling may vary between countries.
Please choose an option that best describes you:
For visitors outside the United States: click here