
Of the patients meeting criteria of drug-related hepatic disorders, 528 were from post-marketing sources and 36 from trials after the SKYLIGHT program.
Table 1. Reported time to first detection in 235/564 cases1
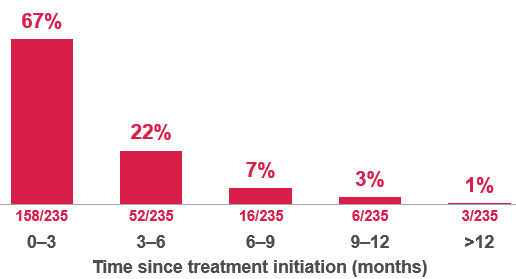
Effect of discontinuation1
Data on file.
Drug-induced Liver Injury (DILI): Current status and future directions for drug development and the post-market setting. A consensus by a CIOMS Working Group. Council for International Organizations of Medical Sciences (CIOMS). Available at: https://cioms.ch/wp-content/uploads/2020/06/CIOMS_DILI_Web_16Jun2020.pdf.
The medical information on this website is for educational purposes only and is intended to provide scientific information about Astellas products. This information is not intended as medical advice or clinical recommendations. This website is for use only by United States residents and licensed healthcare professionals (HCPs) practicing in the United States. Product labeling may vary between countries.
Please choose an option that best describes you:
For visitors outside the United States: click here