
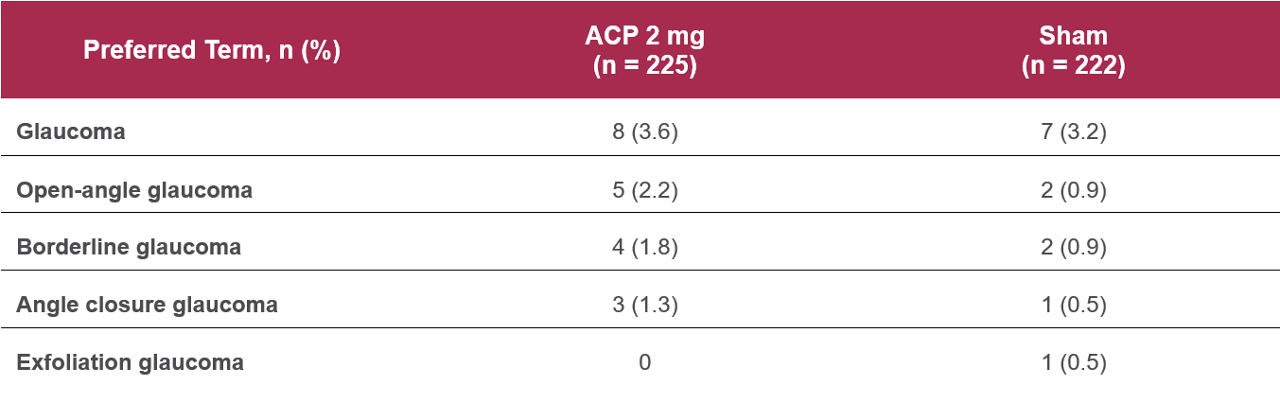
Some patients in the GATHER1 and GATHER2 trials of avacincaptad pegol (ACP) had a prior history of glaucoma in the study eye, but no subgroup safety or efficacy analyses were conducted in these patients.1
ACP was assessed in patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD) in two pivotal studies: GATHER1, a Phase 2/3 clinical trial, and GATHER2, a Phase 3 trial.2,3 Some participants had a baseline medical history of glaucoma in the study eye, which included open-angle glaucoma, normal tension glaucoma, borderline glaucoma, angle-closure glaucoma, and exfoliation glaucoma. No analyses were conducted to evaluate differences in the safety or efficacy of ACP in this patient population.
Table 1. Medical history of glaucoma in study eye at baseline – GATHER11
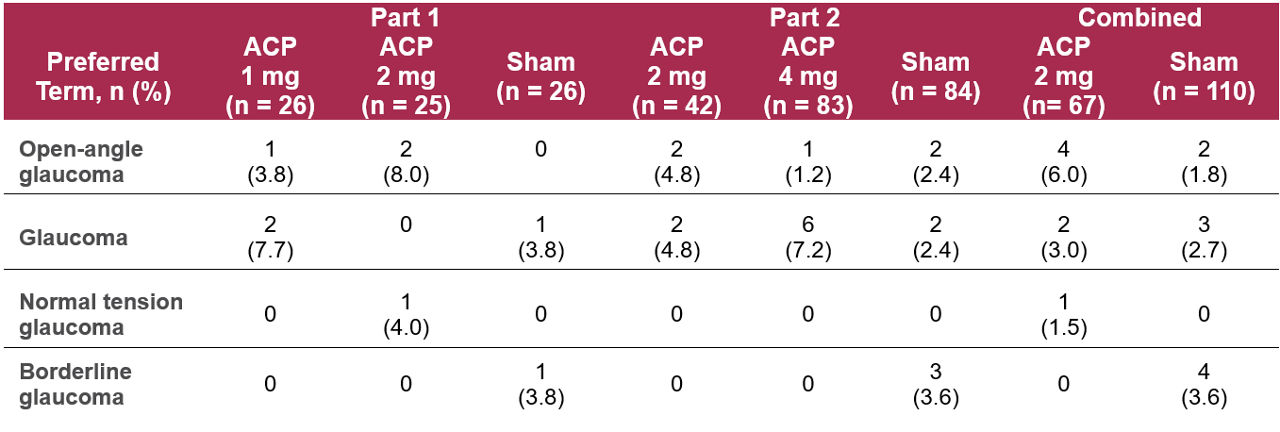
Table 2. Medical history of glaucoma in study eye at baseline – GATHER21
Data on File.
Jaffe GJ, Westby K, Csaky KG, et al. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration A Randomized Pivotal Phase 2/3 Trial. Ophthalmology. 2021;128(4):576-586. https://dx.doi.org/10.1016/j.ophtha.2020.08.027.
Khanani AM, Patel SS, Staurenghi G, et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, phase 3 trial. Lancet. 2023;402(10411):1449-1458. https://dx.doi.org/10.1016/s0140-6736(23)01583-0.
The medical information on this website is for educational purposes only and is intended to provide scientific information about Astellas products. This information is not intended as medical advice or clinical recommendations. This website is for use only by United States residents and licensed healthcare professionals (HCPs) practicing in the United States. Product labeling may vary between countries.
Please choose an option that best describes you:
For visitors outside the United States: click here